The Australian Scleroderma Cohort study was designed by ASIG in 2007, with the aim of creating a detailed national database about people with scleroderma (SSc) and mixed connective tissue disease (MCTD). ASIG also established a bio-bank to collect and store bio specimens to provide novel insights into biomarkers and genetic component of this rare disease. All people with SSc or MCTD attending any of the ASIG centres are offered the opportunity to participate in the ASCS and the biobank.
Data from this ongoing cohort have been stored on a secure database at St Vincent’s Hospital in Melbourne. The ASCS has been recognised as one of the most comprehensive SSc databases in the world and has been instrumental in developing the ASIG research programme which has so far resulted in 97 publications, and supervision of 3 Masters Students and 5 PhD students. The programme has raised the profile of SSc research in Australia and provided training for many rheumatologists in this devastating condition.
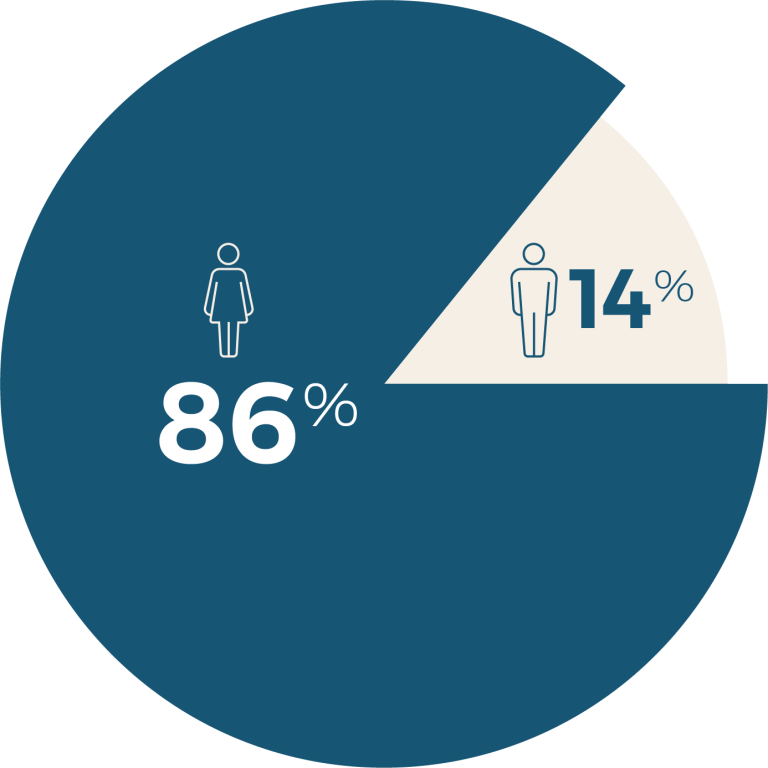
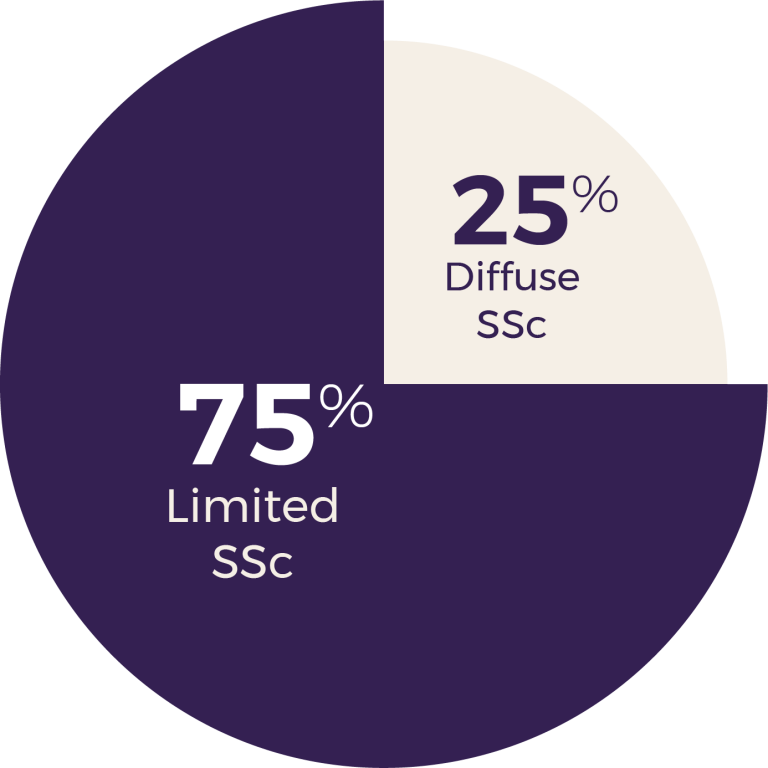
Since 2007, 2,039 patients have enrolled in the ASCS of whom 1,878 meet the ACR/EULAR criteria for diagnosis. The vast majority of patient are Caucasian (90.9%), female (85.6%) and with the limited disease subtype (74.7%), reflecting the epidemiology of scleroderma. The median age of disease onset is 47.1 (IQR 36.3 - 56.9) years, with a median of 7.3 (IQR 2.5 - 15.8) years between disease onset and enrolment in the ASCS.
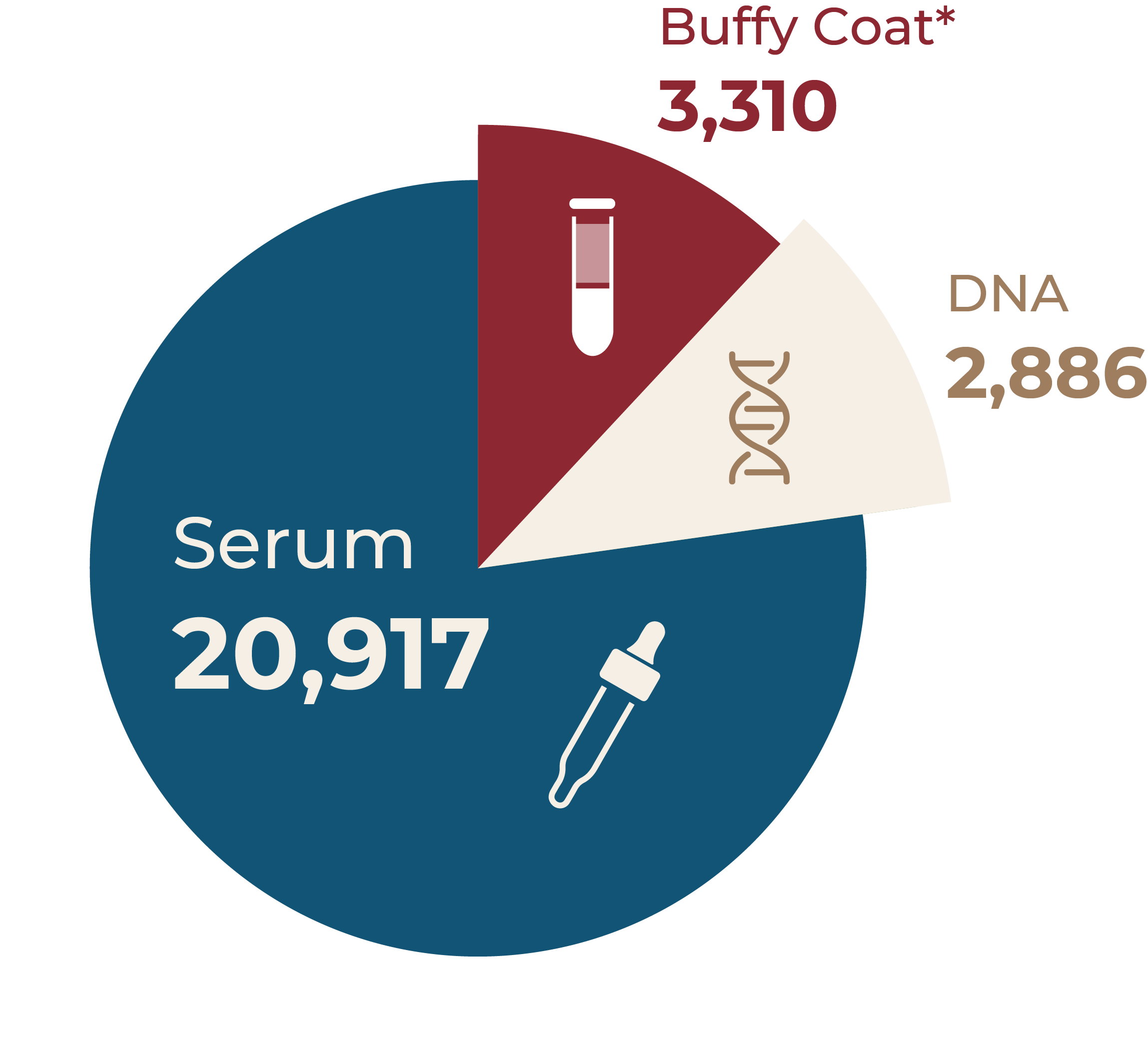
Patients who give informed consent to enrolment in the ASCS are also invited to provide an annual serum sample for storage for future biomarker studies. If they provide additional consent, patients are also invited to submit a single whole blood sample for extraction of DNA for genetic studies. These samples are stored in dedicated -80° freezers at the University of South Australia, North Terrace, Adelaide, South Australia where they are managed by our Biobank Manager, Mahin Moghaddami. Since the establishment of the biobank, 27,113 samples have been collected and stored in the ASCS biobank. These samples represent a valuable resource for collaborative research with researchers nationally and internationally. Work to date includes studies of biomarkers for pulmonary arterial hypertension (PAH) e.g. NT-proBNP, which has led to the development of the ASIG screening algorithm for PAH, and other biomarkers for interstitial lung disease.
We are now planning to expand our biobank to include peripheral blood mononuclear cells, skin biopsies and faecal specimens. This will enable study of cellular and molecular mechanisms involved in the development of SSc and potentially identify targets for new therapeutics.